Dr. Diane Harper was a leading expert responsible for the Phase II and Phase III safety and effectiveness studies which secured the approval of the  human papilloma virus (HPV) vaccines, Gardasil™ and Cervarix™. Dr. Harper also authored many of the published, scholarly papers about the vaccines. She is now the latest in a long string of experts who are pressing the red alert button on the devastating consequences and irrelevancy of these vaccines. Dr. Harper made her surprising confession at the 4th International Converence on Vaccination which took place in Reston, Virginia. Her speech, which was originally intended to promote the benefits of the vaccines, took a 180-degree turn when she chose instead to clean her conscience about the deadly vaccines so she “could sleep at night”. The following is an excerpt from a story by Sarah Cain…
human papilloma virus (HPV) vaccines, Gardasil™ and Cervarix™. Dr. Harper also authored many of the published, scholarly papers about the vaccines. She is now the latest in a long string of experts who are pressing the red alert button on the devastating consequences and irrelevancy of these vaccines. Dr. Harper made her surprising confession at the 4th International Converence on Vaccination which took place in Reston, Virginia. Her speech, which was originally intended to promote the benefits of the vaccines, took a 180-degree turn when she chose instead to clean her conscience about the deadly vaccines so she “could sleep at night”. The following is an excerpt from a story by Sarah Cain…
Category: HPV vaccine related death
Gardasil: Criminal complaint filed in Spain#Vaccines#iBelieve#HPV
By Norma Erickson
![]()
 June 19 2014, Logroño, Spain: Attorney Don Manuel Sáez Ochoa filed a criminal complaint against Merck-Sanofi Pasteur Laboratories, Spanish National Health authorities, and the regional health authorities of the La Rioja province on behalf of Zuriñe Jiménez Guereño and her mother Doña Maria del Carmen Jiménez Guereño for injuries and disabilities suffered by Zuriñe after the administration of Gardasil.
June 19 2014, Logroño, Spain: Attorney Don Manuel Sáez Ochoa filed a criminal complaint against Merck-Sanofi Pasteur Laboratories, Spanish National Health authorities, and the regional health authorities of the La Rioja province on behalf of Zuriñe Jiménez Guereño and her mother Doña Maria del Carmen Jiménez Guereño for injuries and disabilities suffered by Zuriñe after the administration of Gardasil. 
The complaint states that Merck Laboratories failed to use an inert placebo during clinical trials, thereby manipulating data and marketing Gardasil under false pretences. Despite complaints of several young women with similar new medical conditions after Gardasil injections, the Spanish health authorities ignored calls for a moratorium on the use of Gardasil until the safety issues were resolved.
Both regional and national health authorities made no attempt to verify the accuracy of the safety data Merck submitted to gain approval for the widespread administration of Gardasil as a cancer preventative; nor did they make any attempt to inform the public that an already proven safe and effective means of controlling cervical cancer was already in existence.
The complaint goes on to say both national and local health authorities had adequate knowledge regarding the potential harmful effects of Gardasil and chose to recommend administration of the HPV vaccine anyway. The complaint alleges this showed an absolute disregard for the health and well-being of young Spanish girls.
According to the complaint, the attitude of the Merck pharmaceutical company and Spanish health authorities (both national and regional) before, during and after the administration of Gardasil shows they care nothing about the risk to which medical consumers expose themselves whenever Gardasil is used.
The complaint states, prior to administration, no one was concerned about possible adverse reactions to the vaccine. When adverse reactions did occur, those who experienced them were treated with contempt leaving them in a state of helplessness. There was allegedly not one single official inquiry about the girls’ post-Gardasil conditions even though they were healthy prior to being injected with Gardasil.
An outcry from the public calling for a moratorium on the use of Gardasil until safety issues were resolved was ignored by Spanish health authorities. Injections of Gardasil continued despite the damage left behind.
According to Attorney Don Manuel Sáez Ochoa,
(claiming) a possible exemption arguing that they did not know at the time of processing, the dangers of the vaccine (Gardasil) is laughable……Frankly this attitude seems clearly malicious and constitutes the offense of injury as per Artile 149.1 of the Criminal Code that states: To cause another, by any means or process, the loss or worthlessness of an organ or principal member, or a sense, impotence, sterility, severe deformity, or severe somatic or mental illness, shall be punished with imprisonment of six to twelve years.
Charges contained in the official criminal complaint
Merck-Sanofi Pasteur, Spain’s National and Regional (La Rioja) health authorities are charged with the following:
- fraudulent marketing and/or administration of an inadequately tested vaccine;
- failure to inform the public about the potential risks of using Gardasil;
- clear infringement of the right to informed consent;
- ignoring new medical conditions in those who used Gardasil despite the similarity of their symptoms and the relatively short period of time between vaccine administration and the onset of symptoms;
- ignoring established and new scientific evidence illustrating the potential harmful effects of Gardasil ingredients and manufacturing methods;
- callous disregard for those suffering new medical conditions post-Gardasil;
- failure to inform the public that HPV infections are simply one of the risk factors involved in the development of cervical cancer;
- failure to inform the public that 90% of all HPV infections clear on their own without medical intervention;
- failure to inform the publlic about alternative methods of controlling cervical cancer; and
- criminal liability for the injuries resulting from the administration of Gardasil
Even if one assumes that Merck-Sanofi Pasteur and all of the government health officials were not aware of the potential risks and lack of proven benefit of Gardasil, there has been plenty of scientific and medical evidence provided since 2007 when the vaccine was approved for use in Spain.
Given their expertise, all of these people were aware of the fact that there are several methods to control cervical cancer that have already been proven safe and effective.
Zuriñe was a healthy, athletic and academically gifted girl until she received the recommended three doses of Gardasil via an immunization program at her school when she was 13 years old. Three weeks later, she was admitted to the emergency room of her local hospital suffering from a multitude of symptoms including dizziness, fatigue and convulsions.
After receiving no answers from her doctors, the conversations began to include hints of conversion disorder. Her parents decided to take her to a private specialist.
According to this specialist, Dr. Mark Mazzuca:
Zuriñe suffers severe cell disease, oxidative stress linked to a demineralization of her body. To put it graphically, she is an 18 year old girl locked in a cell body of a person over sixty years old. Zuriñe also suffers from hard infield Ortostátiaco Postural Syndrome polyneuropathy revealing a central character. It also presents as liver and bladder inflammation.
Five years after her last Gardasil shot, Zuriñe’s life bears little resemblance to anything she once considered normal. In and out of hospitals dealing with ‘mysterious’ new medical conditions every day. No one knows how much of her normal life she will be able to regain.
Thousands of young women around the world are finding themselves in the same position as Zuriñe. They have gone from being happy, active, and healthy to facing a multitude of autoimmune problems and neurological disorders. For them, the ‘possible’ adverse effects of Gardasil have become an all too harsh and brutal reality.
It is time for those responsible to be held accountable for their actions. Criminal prosecution is quite possibly the only way to accomplish that goal.
Perhaps six to twelve years in prison would remind those responsible what it means to conduct yourself in an ethical manner. Perhaps they would remember that their first duty is to maintain the public health, not destroy it.
On July 30, the Judge decided to open criminal proceedings and investigation of the facts. The first criminal case in Spain regarding Gardasil injuries and potential criminal liability begins.
VACCINATIONS: KNOW THE RISKS#Vaccines#ASD#iBelieve
There is a wide spectrum of vaccine complications, which have been identified and acknowledged in the medical literature and by the Institute of Medicine (IOM), National Academy of Sciences, including:14
- Brain Inflammation/Acute Encephalopathy
- Chronic Nervous System Dysfunction

- Anaphylaxis
- Febrile Seizures
- Guillain Barre Syndrome (GBS)
- Brachial Neuritis;
- Acute and Chronic Arthritis
- Thrombocytopenia
- Smallpox, polio, measles and varicella zoster vaccine strain infection
- Death (smallpox, polio and measles vaccine)
- Shock and “unusual shock-like state”
- Protracted, inconsolable crying
- Syncope
- Deltoid Bursitis
Spain: First case filed against HPV vaccine manufacturers and health authorities#Vaccines#HPV#Health
By Alicia Capilla (President of AAVP)
 AAVP, together with law firm Almodóvar & Jara, filed the first of a long series of lawsuits for damages caused by HPV vaccines. The complaint is filed in the High Court against health authorities and vaccine manufacturers.
AAVP, together with law firm Almodóvar & Jara, filed the first of a long series of lawsuits for damages caused by HPV vaccines. The complaint is filed in the High Court against health authorities and vaccine manufacturers.
The process of trying to find justice now begins for one of the Valencian girls who suffered an adverse reaction after the second shot of Gardasil in 2009. Spanish families whose lives have been adversely impacted by HPV vaccines have organized as the Association of Affected People by HPV Vaccine (AAVP www.aavp.es) to assist others in similar circumstances.
The well documented lawsuit is based on violations of the fundamental right to informed consent prior to medical interventions which all citizens have.
Parents whose daughters are vaccinated with Gardasil are not informed beforehand of the possible risks their daughters may suffer, despite the fact there are numerous reports in Spanish, European and American databases. Furthermore, most of the adverse reactions these girls suffered are included in the brochure/leaflet of the product.
The introduction of HPV vaccines into the market without their real effectiveness being known is another issue. The effectiveness has not sufficiently been proven and will not be demonstrated for decades.
Much of the damage these vaccines are producing is being hidden, despite the fact that pharmacovigilance systems around the world are collecting numerous reports of similar reactions.
Moreover, the Spanish Health Ministry withheld information from affected families by denying that the damage the girls suffered was legitimate and previously known via similar cases being reported to health authorities in various countries.
The Spanish Ministry of Health, Sanofi Pasteur, and Merck, Sharp and Dohme (MSD), producers of Gardasil®, human papillomavirus vaccine, have a responsibility to report accurately and in a timely manner all data available at the time.
Health authorities around the world are trying to deny any causal relationship between HPV vaccines and adverse events occurring after vaccine use. In some cases, authorities say that the new medical conditions are psychological. This is a paradox because if people in different times and different places suffer a similar adverse reaction, it is undeniable that the cause is the vaccine.
The damage these girls have suffered and many continue to suffer were not all included in the leaflet at the time of injection. Some of them were included later. Others continue without being warned.
Based on data that the AAVP has examined, the number of suspected deaths and serious sequelae left behind after human papillomavirus vaccines is completely unacceptable.
As stated in the case filed, health authorities do not investigate the facts and their attitude is favoring manufacturers. They even accused the victims of suffering psychological disorders, which is not true.
This first case will be followed by another four within two months. The firm will continue to file additional cases, not only against Gardasil® but also Cervarix®, the other brand of the HPV vaccinemanufactured by GlaxoSmithKline.
This article, in it’s entirety, is compliments of SaneVax.org.
HPV Vaccine Trials in India: Is Merck above the law?#vaccines#iBelieve#HPV
3 July 2014: As a result of continued investigations regarding clinical trials involving HPV vaccines in India, more allegations of unethical conduct have been lodged against Merck. These allegations pertain to Merck’s recent trials of the new V503 HPV vaccine, a proposed nine valent HPV vaccine. A complete report of the investigation results written by Dr. Anand Rai, Kelly O’Connor, Amoli Tuli, and Anisha Bhattacharya is now a matter of public record. (Link to report)
The additional allegations of multiple ethics violations were added to a currently ongoing Supreme Court Case via an affidavit added as an addendum to the brief previously prepared regarding the ’demonstration projects’ conducted by a U.S NGO PATH in India beginning in 2009. The deaths of subjects during the ’demonstration projects’ resulted in a government-ordered enquiry and suspension of all HPV vaccine trials in India pending further investigation. This suspension was effective as of April 2010 and is still in effect.
Ultimately, the suspension and subsequent investigations allowed advocates in India to take their case all the way to the Supreme Court. The petition for the case was formally admitted by the judges on January 7, 2013. By September 2013, the court had issued notices to all participants involved while the Indian Parliament issued a scathing comment condemning all organizations involved in the so-called ’demonstration projects’ calling them a case of child abuse expressly carried out to create a market for the two companies – Merck and GlaxoSmithKline.
According to new allegations, Merck ignored the suspension of HPV-related clinical trials and continued with a phase III trial of the yet-to-be-approved investigational HPV vaccine called V503, which theoretically adds protection against 5 types of HPV to the ones already included in their current HPV vaccine, Gardasil.
Allegations of Merck’s Misconduct
The supplemental affidavit submitted to the Supreme Court of India states that Merck and/or their representatives committed the following violations of basic human rights:
1. Respondent No. 8, MSD Pharmaceuticals Pvt. Ltd. and its affiliate companies in respect of pre-licensure trials with investigational HPV vaccine V-503.
2. Pre-licensure Phase III trials were carried out at many centres in India. One of these centres was Indore. In Indore this vaccine was administered to 44 children, boys and girls. A field study with the participants was carried out by Dr. Anand Rai and others and is detailed in the Annexure.
3. The timing of this trial was such that vaccination was carried out during the same period when Respondent no. 1, The Ministry of Health and Family Welfare, had asked all HPV related studies to suspend vaccination. As such then this was a violation of the government orders.
4. The Annexure also lists in detail all the problems with this trial on a case by case basis, some of them are summarized below:
a) This clinical trial was not preceded by any trial among adults thereby violating the Drugs and Cosmetics Act. This trial had the approval of the Respondent No. 2, The Drugs Controller General of India (DCGI), who violated the law that his job specifically asked him to uphold. The petitioners have complained to the court that is the second time regarding HPV vaccines the DCGI bestowed this favour on Merck and its associates.
b) The trial used middle men and women acting as agents of the investigator who went around residential areas recruiting subjects in complete violation of all national and international guidelines.
c) The trial was carried out at a government hospital using government stationery and seal even though this was a privately sponsored trial of the company thereby misusing the trust parents place in the governmental vaccination programmes.
d) The parents and children were told that they were getting a successful vaccine from abroad free which would otherwise cost Rs 10,000. This was a blatant lie as this vaccine was not approved for marketing anywhere in the world. Also this constituted undue inducement to recruit children to this trial.
e) None of the participants were informed that they were participating in a clinical trial.
f) Economically vulnerable sections and those from scheduled castes and minorities were recruited for the trial, those who would not be able to afford the vaccine if and when it is marketed; thereby the trial further violated all laws and guidelines in this regard.
g) Merck listed history of allergic reaction that required medical intervention, currently enrolled in another clinical trial, subject is pregnant, subject is immunocompromised or has taken immunosuppressants in the last year, subject has received a marketed HPV vaccine or participated in an HPV vaccine clinical trial and subject has history of positive test for HPV as exclusion criteria for this clinical trial. No examinations of the children who were selected for participation were undertaken prior to administration of the V503 vaccine.
h) There was no placebo involved in this trial, simply three different lots of V503, despite the fact that one purpose of the trials was to detect potential safety issues.
i) From the beginning to end, there was no examination of the children for their health status, and even though the trial was supposedly carried out to determine the safety of the vaccine not even fever post vaccination was recorded.
j) No medical care was provided for the health problems of the children nor were they provided any compensation. The study found many children who continue to suffer new medical problems even as late as 2013. Out of the 12 individuals interviewed, 4 have experienced health problems after the vaccine, such as mood swings, persistent stomach aches, dizziness, late onset of periods and severe anaemia. One boy whose family was interviewed has suffered extreme weight loss.
k) Though the investigation covered only the trial centre at Indore the entire list of trial centres is placed in the supplemental affidavit for information. In all hundreds of children have been affected in India.
One would think all of the complaints filed against PATH and Merck during the ’demonstration projects’ for Gardasil would have caused them to be on their best behavior. But apparently, when the government officials in India called a halt to any trials relating to HPV, Merck thought the decree did not apply to them. Either that, or they believed themselves powerful enough to be above the law.
Whatever the motivation, it appears hundreds more economically disadvantaged children and their families were put at risk by their participation in a clinical trial they were not told they were taking part in.
Compound this disregard for informed consent with participants being told they were priviledged to receive a successful and very expensive vaccine from overseas. Nevermind the fact that this ’successful vaccine’ had not been approved for use in any country in the world.
The people of India are not the only ones who may suffer the effects of this particular set of clinical trials. What ’results’ will Merck report to the rest of the world on this set of trials where the exclusion criteria was apparently ignored?
What ’results’ will Merck report to the rest of the world when no adverse events were recorded? One can certainly assume the adverse events which apparently were not recorded will no longer exist.
Will pretending these new medical conditions did not happen allow Merck to report their new V503 HPV vaccine is safe?
Is Merck above the law?
Will Merck be allowed to ignore the international laws protecting the basic right of informed consent prior to participation in clinical trials? Will Merck be allowed to continue to exploit economically disadvantaged people then extrapolate the resulting ’conclusions’ to the entire population of the world?
One can only hope the Supreme Court in India decides to protect the health and well-being of their young people by responding to the complaints of the organizations who put forth the allegations in a manner which will stop these types of abuses from ever happening in their country again.
By doing so, India’s Supreme Court Judges could help protect all of the world’s young people.
The Supreme Court of India has scheduled the final hearing of this case for August 12, 2014. The Court’s decision should be rendered on that date.
Whatever the ultimate outcome, advocates around the world will be watching.
Show Me The Science#vaccines#Health#iBelieve
We have compiled this catalogue of science to help parents, lawmakers, medical practitioners and scientists understand several important points about the vaccine issue:
- there is abundant science published in mainstream medical and scientific journals suggesting cause for concern about the safety of vaccines;
- the vaccine debate is not a debate between parents and doctors but rather amongst scientists with opposing views;
- vaccines may be linked to a host of chronic illnesses and conditions such as asthma, allergies, learning disabilities, behavioral problems, autism, unexplained infant death and autoimmune diseases such as diabetes, rheumatoid arthritis, lupus, MS, and others;
- there are connections between the gut, immune, and neurological issues often seen in vaccine injuries.

Is The Epidemic of Sudden Infant Deaths A Medically Induced ‘Syndrome’?#vaccines#iBelieve#Health
A new study published in Current Medicine and Chemistry titled, “Sudden infant death following hexavalent vaccination: a neuropathologic study,” lends support for the long theorized link between an ever-expanding number of infant vaccines and Sudden Infant Death Syndrome (SIDS).
The fact that the peak age for SIDS is 2–4 months, which coincides with the introduction of 11 shots containing 16 vaccines (within the US immunization schedule ), is so obvious a cause for concern, that even the CDC has been compelled to address the seeming ‘coincidence’ directly:
), is so obvious a cause for concern, that even the CDC has been compelled to address the seeming ‘coincidence’ directly:
“From 2 to 4 months old, babies begin their primary course of vaccinations. This is also the peak age for sudden infant death syndrome (SIDS). The timing of these two events has led some people to believe they might be related…With babies receiving multiple doses of vaccines during their first year of life and SIDS being the leading cause of death in babies between one month and one year of age, CDC has led research studies to look for possible linkage.”
Unsurprisingly, the CDC, whose pro-vaccine agenda is glaringly oblivious to the 100+ documented serious, unintended adverse effects of vaccines as evidenced in the biomedical literature, claims extensive research they commissioned has found vaccines do not cause SIDS. Despite the CDC’s dismissal, infant mortality rates are highest among countries that administer the most vaccines within the most vulnerable developmental window of infanthood. A 2011 study published in Human & Experimental Toxicology, for instance, observed that “The US childhood immunization schedule specifies 26 vaccine doses for infants aged less than 1 year—the most in the world—yet 33 nations have lower IMRs [infant mortality rates].” They found that across the 34 nations analyzed “a high statistically significant correlation between increasing number of vaccine doses and increasing infant mortality rates, with r = 0.992 (p = 0.0009).”
specifies 26 vaccine doses for infants aged less than 1 year—the most in the world—yet 33 nations have lower IMRs [infant mortality rates].” They found that across the 34 nations analyzed “a high statistically significant correlation between increasing number of vaccine doses and increasing infant mortality rates, with r = 0.992 (p = 0.0009).”
Also, a recent study published in Vaccine titled, “Co-administration of live measles and yellow fever vaccines and inactivated pentavalent vaccines is associated with increased mortality compared with measles and yellow fever vaccines only” found multiple infant vaccines dramatically increased the risk of mortality in a trial conducted in the West African country of Guinea-Bissau…
HPV Vaccines: Time for Science#iBelieve#vaccines#HPV
By Norma Erickson
According to the World Health Organization (WHO), as of January 2014 fifty-two countries have included HPV vaccines, either Gardasil or Cervarix, in their national immunization programs. During the last five years, the SaneVax Team has been contacted by representatives from 24 of those countries who are seeking to understand the vast array of new medical conditions occurring in the wake of these programs.
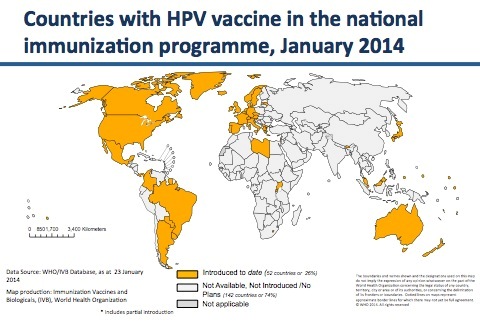 (Note: SaneVax has been contacted by multiple people in Australia, Argentina, Belgium, Canada, Colombia, Denmark, Finland, France, Germany, India, Ireland, Israel, Japan, Malaysia, Morocco, New Zealand, Norway, Peru, Scotland, Spain, Sweden, Switzerland, the United Kingdom and the United States – all requesting further information on HPV vaccines and vaccination programs.)
(Note: SaneVax has been contacted by multiple people in Australia, Argentina, Belgium, Canada, Colombia, Denmark, Finland, France, Germany, India, Ireland, Israel, Japan, Malaysia, Morocco, New Zealand, Norway, Peru, Scotland, Spain, Sweden, Switzerland, the United Kingdom and the United States – all requesting further information on HPV vaccines and vaccination programs.)
Why would so many countries contact an organization dedicated to the promotion of only safe, affordable, necessary and effective vaccines and vaccination programs in such a relatively short period of time? Let’s take a look at the science.
In 2006, when the FDA approved the first HPV vaccine (Gardasil) for cervical cancer prevention they should have known that HPV does not cause cervical cancer without other risk factors being involved. They should have known that the endpoints chosen to evaluate the efficacy of the vaccine were not sufficient to prove future cervical cancer prevention because ASCUS and CIN1/2/3 frequently resolve on their own, or can be detected by currently available tests (pap smears) and treated safely and effectively prior to progression to cervical cancer. They should have known that allowing the manufacturer to use a reactive aluminum adjuvant as a ’control solution’instead of an inert placebo could potentially mask adverse effects making the vaccine appear more safe than it actually is.
Any one of these facts should have raised questions about the practicality of utilizing HPV vaccines in any cervical cancer prevention program. Apparently the facts above were either not known, or they were ignored. Gardasil was awarded fast-track approval despite the fact there was no cervical cancer crisis in the United States. A short time later, Cervarix was approved for the same indication.
Since health officials at the FDA are apparently ignoring scientific facts medical consumers need to be aware of the science, or lack therof, behind HPV vaccines.
Does HPV cause cervical cancer?
 According to WHO, only 0.15% of people exposed to any high risk HPV types will ever develop cervical cancer. We do not have a publication date on the chart to the right, so let’s assume this is new knowledge, information which the FDA officials responsible for the fast-track approval of Gardasil did not have when they made the decision to approve the vaccines for cervical cancer prevention.
According to WHO, only 0.15% of people exposed to any high risk HPV types will ever develop cervical cancer. We do not have a publication date on the chart to the right, so let’s assume this is new knowledge, information which the FDA officials responsible for the fast-track approval of Gardasil did not have when they made the decision to approve the vaccines for cervical cancer prevention.
Medical consumers need to know that according to the World Health Organization 99.85% of those exposed to oncogenic types of HPV will never develop cervical cancer.
IF the FDA did not know this prior to approving HPV vaccines to ’prevent’ cervical cancer, they are most assuredly know it now. Consider the following quote from an FDA/CBER (Center for Biologics Evaluation and Research) meeting held on September 19, 2012 to discuss the use of human tumor cells in vaccine production (verify on pages 91-92 of this transcript):
“However, even tumor induction by acute oncogenic viruses requires additional oncogenic events. The best example is the Human Papillomavirus, where even infection with a high risk of Papillomavirus types is not sufficient to induce cervical cancer. Mutation in other genes and perhaps also epigenetic events are required. If a single infection-induced cervical cancer after infection with HPV, say, 16, then teenagers will be getting cervical cancer. And as we know, cervical cancer is a disease of older people”.
So, there you have it – even infection with a high risk strain of papillomavirus types is not sufficient to induce cervical cancer. Since at least September 2012, FDA officials knew infections with high-risk HPV types will not induce cancer without genetic mutations and/or other epigenetic (relating to or arising from nongenetic influences on gene expression) events occurring also.
Did this cause the FDA or CDC to re-examine the usefulness of HPV vaccines? No.
Were clinical trial endpoints valid?
Prior to the marketing push for HPV vaccines, CIN1/2/3 were known as abnormal cells – something that needed to be observed until treatment was required. Now, they are almost always referred to as ‘pre-cancerous’ lesions. This serves no purpose other than to strike fear into the heart of almost any woman on the face of the planet. The nature of the abnormal cells has not changed, simply the terminology. No mention is made of the fact that CIN1, CIN2 and often CIN3 abnormal cells revert to normal cells without medical intervention.
Not many people are aware of the fact that most CIN1 lesions go away on their own within two years. 25-50% of CIN2 lesions regress on their own within the same two year time frame. According to the International Agency for Research on Cancer (IARC), World Health Organization, the results of a pooled analysis of studies published between 1950 and 1993 indicated only 12% of CIN3 lesions progress to invasive cervical cancer.
Consider the following quote from Chapter 2 of the IARC’s COLPOSCOPY AND TREATMENT OF CERVICAL INTRAEPITHELIAL NEOPLASIA: A BEGINNER’S MANUAL states:
“Despite women’s frequent exposure to HPV, development of cervical neoplasia is uncommon. Most cervical abnormalities caused by HPV infection are unlikely to progress to high-grade CIN or cervical cancer, as most of them regress by themselves. The long time frame between initial infection and overt disease indicates that several cofactors (e.g., genetic differences, hormonal effects, micronutrient deficiencies, smoking, or chronic inflammation) may be necessary for disease progression. Spontaneous regression of CIN may also indicate that many women may not be exposed to these cofactors.”
Please note this manual was designed to teach medical and nursing personnel in developing countries where diagnostic and therapeutic expertise is not readily available. In other words, the progression from HPV exposure to potential development of cervical cancer is similar in both developing countries and developed countries. It also indicates that several cofactors (risk factors) may be needed for HPV exposure to progress to abnormal lesions, much less cervical cancer.
Surely, FDA officials were aware of the information contained in a beginner’s manual to treatment of so-called ‘precancerous’ lesions published by the World Health Organization. If not, they certainly should have been. Nevertheless, they allowed these very same abnormal cells which typically revert to normal on their own to be used to evaluate the efficacy of HPV vaccines against cervical cancer.
Medical consumers need to know, according to WHO only 1% of CIN1, 1.5% of CIN2 and 12% of CIN3 progress to invasive cervical cancer. This is in developing countries. In countries where pap smears are routinely administered the percentages should be much lower. Could using these abnormal cells as endpoints in clinical trials artificially inflate the predicted efficacy of HPV vaccines against cervical cancer? Absolutely!
Why not use a true placebo during clinical trials?
When conducting scientific experiments to evaluate a new substance, standard practice is to have at least two groups – one group using the substance being examined and the other using a placebo. This way, the scientific team can evaluate the effects and safety of the new substance by comparing both groups’ results at the end of the study.
The definition of placebo is “a substance having no pharmacological effect, but administered as a control in testing experimentally or clinically the efficacy of a biologically active preparation.”
There is no doubt about vaccines being a biologically active preparation. But, how about aluminum – one of the major ingredients in the proprietary AAHS solution which Merck was allowed to use as a control solution in the vast majority of clinical trials for Gardasil?
A quick search of PubMed, using the terms ‘aluminum toxicity human’ returns 1620 papers on studies conducted as early as 1966. If you narrow the search to ‘injected aluminum toxicity’ you come up with 116 papers published as early as 1974. Refine the search even further to ‘injected aluminum toxicity human’ and you still get 31 scientific papers published between 1979 and 2014.
It is extremely difficult to believe the FDA officials responsible for reviewing Gardasil’s application for approval did not know that aluminum compounds were potentially toxic to humans. Yet, they accepted the use of a proprietary (meaning no one knows what was included other than aluminum) AAHS aluminum adjuvant as a control solution instead of an inert placebo.
FDA officials knew, or should have known, that clinical trials conducted in this manner proved nothing other than the fact that Gardasil was no less dangerous than the new AAHS adjuvant which had never been tested for safety in humans. FDA officials ignored the fact that over 70% of all clinical trial participants reported new medical conditions after the trials. (Verify here) One can only speculate as to why this fact did not raise a huge red flag, but apparently it did not.
The FDA could certainly not use the excuse that it would be unethical to withhold vaccination from any of the clinical trial participants because there was currently no ‘comparable’ vaccine available. Why then, did they not require a real placebo?
Is the FDA ignoring new scientific evidence?
Below is a list of scientific information presented to government health officials around the world. None of the presentations below have brought forth a response from FDA officials. Please keep in mind this is by no means a complete list; it is simply a sample:
- February 2014, information presented to Japanese Government health officials by Dr. Sin Hang Lee, Director Milford Medical Laboratory, Inc. (Dr. Lee’s presentation in Tokyo)
- February and May 2014, information presented at a meeting of Japanese Senators by Lucija Tomljenovic, PhD; her and Professor Christopher Shaw presented similar data to health officials in France (Lucija Tomljenovic’s presentation in Tokyo)
- May 2014, presentation to French health officials by Christopher Exley PhD (Chris Exley’s presentation in Paris)
- December 2012, Professor Joe Cummins, Institute of Science in Society, writes DNA Contamination in HPV Vaccines.
How long are medical consumers supposed to put their faith in an agency that repeatedly ignores standard scientific methodology, not to mention scientific evidence? Medical consumers need to remember every single medication that has ever been pulled from the market was first approved ‘safe and effective’ by the FDA. Can you trust them with your life?
Remember – Research before Consent – you can’t un-vaccinate.

