By Norma Erickson
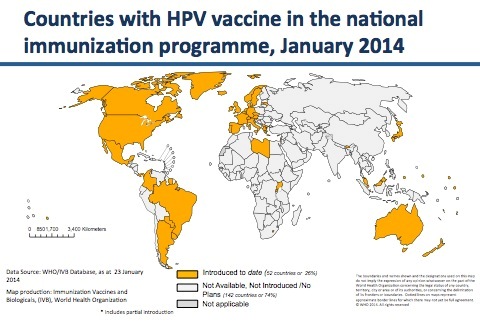
According to the World Health Organization (WHO), as of January 2014 fifty-two countries have included HPV vaccines, either Gardasil or Cervarix, in their national immunization programs. During the last five years, the SaneVax Team has been contacted by representatives from 24 of those countries who are seeking to understand the vast array of new medical conditions occurring in the wake of these programs.
 (Note: SaneVax has been contacted by multiple people in Australia, Argentina, Belgium, Canada, Colombia, Denmark, Finland, France, Germany, India, Ireland, Israel, Japan, Malaysia, Morocco, New Zealand, Norway, Peru, Scotland, Spain, Sweden, Switzerland, the United Kingdom and the United States – all requesting further information on HPV vaccines and vaccination programs.)
(Note: SaneVax has been contacted by multiple people in Australia, Argentina, Belgium, Canada, Colombia, Denmark, Finland, France, Germany, India, Ireland, Israel, Japan, Malaysia, Morocco, New Zealand, Norway, Peru, Scotland, Spain, Sweden, Switzerland, the United Kingdom and the United States – all requesting further information on HPV vaccines and vaccination programs.)
Why would so many countries contact an organization dedicated to the promotion of only safe, affordable, necessary and effective vaccines and vaccination programs in such a relatively short period of time? Let’s take a look at the science.
In 2006, when the FDA approved the first HPV vaccine (Gardasil) for cervical cancer prevention they should have known that HPV does not cause cervical cancer without other risk factors being involved. They should have known that the endpoints chosen to evaluate the efficacy of the vaccine were not sufficient to prove future cervical cancer prevention because ASCUS and CIN1/2/3 frequently resolve on their own, or can be detected by currently available tests (pap smears) and treated safely and effectively prior to progression to cervical cancer. They should have known that allowing the manufacturer to use a reactive aluminum adjuvant as a ’control solution’instead of an inert placebo could potentially mask adverse effects making the vaccine appear more safe than it actually is.
Any one of these facts should have raised questions about the practicality of utilizing HPV vaccines in any cervical cancer prevention program. Apparently the facts above were either not known, or they were ignored. Gardasil was awarded fast-track approval despite the fact there was no cervical cancer crisis in the United States. A short time later, Cervarix was approved for the same indication.
Since health officials at the FDA are apparently ignoring scientific facts medical consumers need to be aware of the science, or lack therof, behind HPV vaccines.
Does HPV cause cervical cancer?
 According to WHO, only 0.15% of people exposed to any high risk HPV types will ever develop cervical cancer. We do not have a publication date on the chart to the right, so let’s assume this is new knowledge, information which the FDA officials responsible for the fast-track approval of Gardasil did not have when they made the decision to approve the vaccines for cervical cancer prevention.
According to WHO, only 0.15% of people exposed to any high risk HPV types will ever develop cervical cancer. We do not have a publication date on the chart to the right, so let’s assume this is new knowledge, information which the FDA officials responsible for the fast-track approval of Gardasil did not have when they made the decision to approve the vaccines for cervical cancer prevention.
Medical consumers need to know that according to the World Health Organization 99.85% of those exposed to oncogenic types of HPV will never develop cervical cancer.
IF the FDA did not know this prior to approving HPV vaccines to ’prevent’ cervical cancer, they are most assuredly know it now. Consider the following quote from an FDA/CBER (Center for Biologics Evaluation and Research) meeting held on September 19, 2012 to discuss the use of human tumor cells in vaccine production (verify on pages 91-92 of this transcript):
“However, even tumor induction by acute oncogenic viruses requires additional oncogenic events. The best example is the Human Papillomavirus, where even infection with a high risk of Papillomavirus types is not sufficient to induce cervical cancer. Mutation in other genes and perhaps also epigenetic events are required. If a single infection-induced cervical cancer after infection with HPV, say, 16, then teenagers will be getting cervical cancer. And as we know, cervical cancer is a disease of older people”.
So, there you have it – even infection with a high risk strain of papillomavirus types is not sufficient to induce cervical cancer. Since at least September 2012, FDA officials knew infections with high-risk HPV types will not induce cancer without genetic mutations and/or other epigenetic (relating to or arising from nongenetic influences on gene expression) events occurring also.
Did this cause the FDA or CDC to re-examine the usefulness of HPV vaccines? No.
Were clinical trial endpoints valid?
Prior to the marketing push for HPV vaccines, CIN1/2/3 were known as abnormal cells – something that needed to be observed until treatment was required. Now, they are almost always referred to as ‘pre-cancerous’ lesions. This serves no purpose other than to strike fear into the heart of almost any woman on the face of the planet. The nature of the abnormal cells has not changed, simply the terminology. No mention is made of the fact that CIN1, CIN2 and often CIN3 abnormal cells revert to normal cells without medical intervention.
Not many people are aware of the fact that most CIN1 lesions go away on their own within two years. 25-50% of CIN2 lesions regress on their own within the same two year time frame. According to the International Agency for Research on Cancer (IARC), World Health Organization, the results of a pooled analysis of studies published between 1950 and 1993 indicated only 12% of CIN3 lesions progress to invasive cervical cancer.
Consider the following quote from Chapter 2 of the IARC’s COLPOSCOPY AND TREATMENT OF CERVICAL INTRAEPITHELIAL NEOPLASIA: A BEGINNER’S MANUAL states:
“Despite women’s frequent exposure to HPV, development of cervical neoplasia is uncommon. Most cervical abnormalities caused by HPV infection are unlikely to progress to high-grade CIN or cervical cancer, as most of them regress by themselves. The long time frame between initial infection and overt disease indicates that several cofactors (e.g., genetic differences, hormonal effects, micronutrient deficiencies, smoking, or chronic inflammation) may be necessary for disease progression. Spontaneous regression of CIN may also indicate that many women may not be exposed to these cofactors.”
Please note this manual was designed to teach medical and nursing personnel in developing countries where diagnostic and therapeutic expertise is not readily available. In other words, the progression from HPV exposure to potential development of cervical cancer is similar in both developing countries and developed countries. It also indicates that several cofactors (risk factors) may be needed for HPV exposure to progress to abnormal lesions, much less cervical cancer.
Surely, FDA officials were aware of the information contained in a beginner’s manual to treatment of so-called ‘precancerous’ lesions published by the World Health Organization. If not, they certainly should have been. Nevertheless, they allowed these very same abnormal cells which typically revert to normal on their own to be used to evaluate the efficacy of HPV vaccines against cervical cancer.
Medical consumers need to know, according to WHO only 1% of CIN1, 1.5% of CIN2 and 12% of CIN3 progress to invasive cervical cancer. This is in developing countries. In countries where pap smears are routinely administered the percentages should be much lower. Could using these abnormal cells as endpoints in clinical trials artificially inflate the predicted efficacy of HPV vaccines against cervical cancer? Absolutely!
Why not use a true placebo during clinical trials?
When conducting scientific experiments to evaluate a new substance, standard practice is to have at least two groups – one group using the substance being examined and the other using a placebo. This way, the scientific team can evaluate the effects and safety of the new substance by comparing both groups’ results at the end of the study.
The definition of placebo is “a substance having no pharmacological effect, but administered as a control in testing experimentally or clinically the efficacy of a biologically active preparation.”
There is no doubt about vaccines being a biologically active preparation. But, how about aluminum – one of the major ingredients in the proprietary AAHS solution which Merck was allowed to use as a control solution in the vast majority of clinical trials for Gardasil?
A quick search of PubMed, using the terms ‘aluminum toxicity human’ returns 1620 papers on studies conducted as early as 1966. If you narrow the search to ‘injected aluminum toxicity’ you come up with 116 papers published as early as 1974. Refine the search even further to ‘injected aluminum toxicity human’ and you still get 31 scientific papers published between 1979 and 2014.
It is extremely difficult to believe the FDA officials responsible for reviewing Gardasil’s application for approval did not know that aluminum compounds were potentially toxic to humans. Yet, they accepted the use of a proprietary (meaning no one knows what was included other than aluminum) AAHS aluminum adjuvant as a control solution instead of an inert placebo.
FDA officials knew, or should have known, that clinical trials conducted in this manner proved nothing other than the fact that Gardasil was no less dangerous than the new AAHS adjuvant which had never been tested for safety in humans. FDA officials ignored the fact that over 70% of all clinical trial participants reported new medical conditions after the trials. (Verify here) One can only speculate as to why this fact did not raise a huge red flag, but apparently it did not.
The FDA could certainly not use the excuse that it would be unethical to withhold vaccination from any of the clinical trial participants because there was currently no ‘comparable’ vaccine available. Why then, did they not require a real placebo?
Is the FDA ignoring new scientific evidence?
Below is a list of scientific information presented to government health officials around the world. None of the presentations below have brought forth a response from FDA officials. Please keep in mind this is by no means a complete list; it is simply a sample:
- February 2014, information presented to Japanese Government health officials by Dr. Sin Hang Lee, Director Milford Medical Laboratory, Inc. (Dr. Lee’s presentation in Tokyo)
- February and May 2014, information presented at a meeting of Japanese Senators by Lucija Tomljenovic, PhD; her and Professor Christopher Shaw presented similar data to health officials in France (Lucija Tomljenovic’s presentation in Tokyo)
- May 2014, presentation to French health officials by Christopher Exley PhD (Chris Exley’s presentation in Paris)
- December 2012, Professor Joe Cummins, Institute of Science in Society, writes DNA Contamination in HPV Vaccines.
How long are medical consumers supposed to put their faith in an agency that repeatedly ignores standard scientific methodology, not to mention scientific evidence? Medical consumers need to remember every single medication that has ever been pulled from the market was first approved ‘safe and effective’ by the FDA. Can you trust them with your life?
Remember – Research before Consent – you can’t un-vaccinate.











